





NaN / 0
Commercial automatic Water softener resin filter Boiler Industrial water softener Underground well water filtration
Get Latest Price
US$ 43
≥1 Sets
Quick Details
Core Components:
Softener tank, Softener valve
Place of Origin:
Shaanxi, China
Brand Name:
APS
Material:
Carton, FRP/Stainless steel softener tank
Product Details

Product Overview
0.3-100 Tons/hour
Commercial automatic Water softener resin filter
Commercial automatic Water softener resin filter Boiler Industrial water softener Underground well water filtration
Water Softener is called as ion exchange water softeners. Its principle is using sodium cation exchange resin to remove calcium and magnesium ions in water and reduce raw water hardness in order to soften hard water and avoid carbonates fouling in the pipeline, container and boiler and so on.
Working Principle:
Water softeners operate on a simple principle: Calcium and magnesium ions in the water switch places with more desirable ions, usually sodium. The exchange eliminates both of the problems of hard water because sodium doesn't precipitate out in pipes or react badly with soap. The amount of sodium this process adds to your water is quite small -- less than 12.5 milligrams per 8-ounce (237-milliliter) glass, well below the standard set by the food and drug administration for "very low sodium".
Input & Output water quality:
Input water hardness: ≤ 8mmol/L (8000ppm)
Products water hardness: ≤ 0.03mmol/L (30ppm)
Products water hardness: ≤ 0.03mmol/L (30ppm)
Product Paramenters

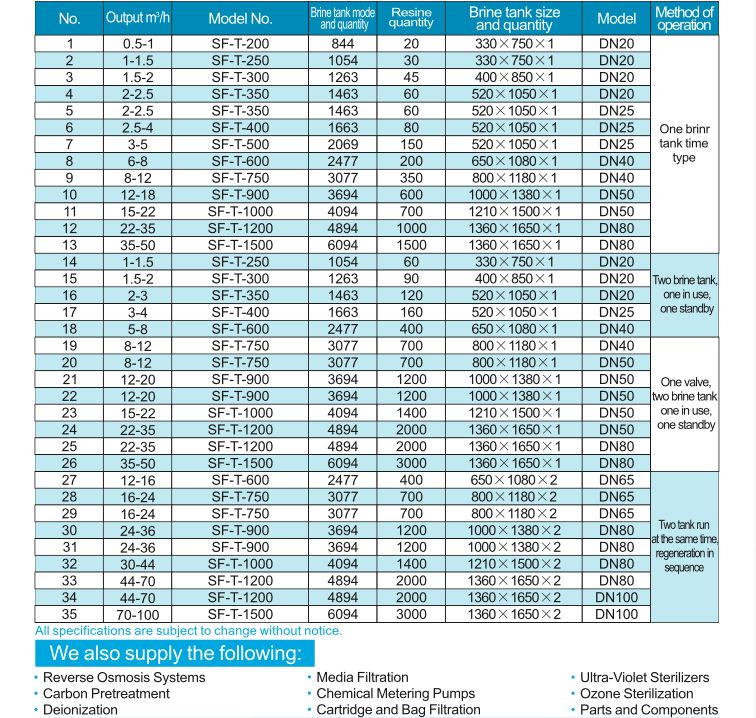
Big Capacity Water Softener

Products photo
APS 300L/hour Automatic water softener
One softener tank
APS 2000L/hour Automatic water softener
Two softener tank, one work one standby
APS 6 tons/hour automatic water softener
One softener tank
APS 10 tons /hour Automatic water softener
One softener tank
APS 1000L/hour Automatic water softener
One valve.
Two softener tank, one work one standby
APS 4000L/hour Automatic water softener
Two valve. Two softener tank. Two brine tank.
APS Stainless steel filter sets
Sand filter + Active carbon filter + Water softener
APS FRP filter sets
Sand filter + Active carbon filter + Water softener
APS 2 stage filter sets
Sand filter + Water softener
APS 3 stage filter sets
Sand filter + Active carbon filter + Water softener
Sand filter + Active carbon filter + Water softener
APS 2 softener tank + 1 brine tank
APS 2 softener tank + 2 brine tank
APS Filter sets + softener
APS 10 tons /hour Water softener
Front view
Front view
APS 10 tons /hour Water softener
Side video
APS 10 tons /hour Water softener
with stainless steel water tank
with stainless steel water tank
APS 10 tons/day
Water softener
Two softener tank
alternate
regenerate
APS 60 tons/day Water softener
Two softener tank alternate regenerate
APS 40 tons/day Water softener
Two softener tank alternate regenerate
APS Softener spare parts in stock
Softener tank
APS Softener spare parts in stock
Brine tank
APS Softener spare parts in stock
Resin
Products Package
APS water treatment equipment package
Delivery & Container loading
Hot Searches








